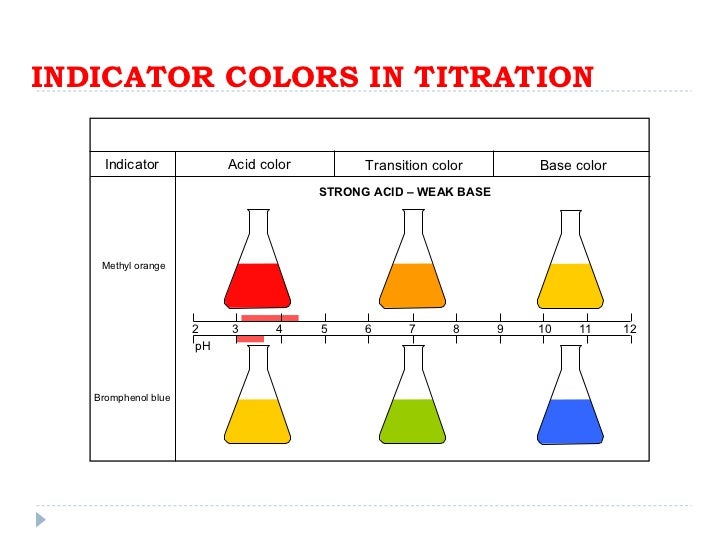
Acid Base Titration Indicator Example . Choosing the best indicator for different titrations depending on the ph at the equivalence point. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Often, an indicator is used to signal the. The analyte (titrand) is the solution with an unknown molarity. Substances such as phenolphthalein, which can. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte.
from mungfali.com
Choosing the best indicator for different titrations depending on the ph at the equivalence point. Often, an indicator is used to signal the. The analyte (titrand) is the solution with an unknown molarity. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Substances such as phenolphthalein, which can.
Acid Base Titration Indicator
Acid Base Titration Indicator Example Often, an indicator is used to signal the. Often, an indicator is used to signal the. The analyte (titrand) is the solution with an unknown molarity. Substances such as phenolphthalein, which can. Choosing the best indicator for different titrations depending on the ph at the equivalence point. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Acid Base Titration Indicator Example Often, an indicator is used to signal the. Choosing the best indicator for different titrations depending on the ph at the equivalence point. Substances such as phenolphthalein, which can. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10. Acid Base Titration Indicator Example.
From glossary.periodni.com
Indicator Chemistry Dictionary & Glossary Acid Base Titration Indicator Example The analyte (titrand) is the solution with an unknown molarity. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. Often, an indicator is used to signal the. Substances. Acid Base Titration Indicator Example.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Acid Base Titration Indicator Example The analyte (titrand) is the solution with an unknown molarity. Often, an indicator is used to signal the. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. Substances. Acid Base Titration Indicator Example.
From mungfali.com
Acid Base Titration Calculation Acid Base Titration Indicator Example Often, an indicator is used to signal the. Choosing the best indicator for different titrations depending on the ph at the equivalence point. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. Substances such as phenolphthalein, which can. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10. Acid Base Titration Indicator Example.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Acid Base Titration Indicator Example Often, an indicator is used to signal the. The analyte (titrand) is the solution with an unknown molarity. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. Choosing the best indicator for different titrations depending on the ph at the equivalence point. Substances such as phenolphthalein, which can. In more basic solutions where. Acid Base Titration Indicator Example.
From mungfali.com
Acid Base Titration Indicator Acid Base Titration Indicator Example The analyte (titrand) is the solution with an unknown molarity. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. Substances such as phenolphthalein, which can. Choosing the best indicator for different titrations depending on the ph at the equivalence point. Often, an indicator is used to signal the. In more basic solutions where. Acid Base Titration Indicator Example.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Acid Base Titration Indicator Example Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. Substances such as phenolphthalein, which can. The analyte (titrand) is the solution with an unknown molarity. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Often, an indicator. Acid Base Titration Indicator Example.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Acid Base Titration Indicator Example Choosing the best indicator for different titrations depending on the ph at the equivalence point. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. Often, an indicator is used to signal the. Substances such as phenolphthalein, which can. The analyte (titrand) is the solution with an unknown molarity. In more basic solutions where. Acid Base Titration Indicator Example.
From mungfali.com
Acid Base Titration Indicator Acid Base Titration Indicator Example The analyte (titrand) is the solution with an unknown molarity. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. Often, an indicator is used to signal the. Choosing the best indicator for different titrations depending on the ph at the equivalence point. Substances such as phenolphthalein, which can. In more basic solutions where. Acid Base Titration Indicator Example.
From saylordotorg.github.io
AcidBase Titrations Acid Base Titration Indicator Example In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. The analyte (titrand) is the solution with an unknown molarity. Choosing the best indicator for different titrations depending on. Acid Base Titration Indicator Example.
From www.slideshare.net
Acid base titration Acid Base Titration Indicator Example Substances such as phenolphthalein, which can. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Choosing the best indicator for different titrations depending on the ph at the. Acid Base Titration Indicator Example.
From www.youtube.com
Acids and Bases Choosing an Indicator for Titration YouTube Acid Base Titration Indicator Example Often, an indicator is used to signal the. Choosing the best indicator for different titrations depending on the ph at the equivalence point. The analyte (titrand) is the solution with an unknown molarity. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. Substances such as phenolphthalein, which can. In more basic solutions where. Acid Base Titration Indicator Example.
From mungfali.com
Acid Base Titration Indicator Acid Base Titration Indicator Example Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. The analyte (titrand) is the solution with an unknown molarity. Substances such as phenolphthalein, which can. Often, an indicator. Acid Base Titration Indicator Example.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid Base Titration Indicator Example The analyte (titrand) is the solution with an unknown molarity. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. Choosing the best indicator for different titrations depending on the ph at the equivalence point. Often, an indicator is used to signal the. Substances such as phenolphthalein, which can. In more basic solutions where. Acid Base Titration Indicator Example.
From www.sliderbase.com
Titration Acid Base Titration Indicator Example Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. The analyte (titrand) is the solution with an unknown molarity. Often, an indicator is used to signal the. Substances such as phenolphthalein, which can. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3),. Acid Base Titration Indicator Example.
From psu.pb.unizin.org
14.7 AcidBase Titrations (2018) Chemistry 112 Chapters 1217 of Acid Base Titration Indicator Example In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. Substances such as phenolphthalein, which can. Often, an indicator is used to signal the. Choosing the best indicator for. Acid Base Titration Indicator Example.
From mungfali.com
Acid Base Titration Indicator Acid Base Titration Indicator Example Often, an indicator is used to signal the. Substances such as phenolphthalein, which can. Knowing the volume of titrant added allows us to determine the concentration of the unknown analyte. The analyte (titrand) is the solution with an unknown molarity. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3),. Acid Base Titration Indicator Example.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Acid Base Titration Indicator Example Choosing the best indicator for different titrations depending on the ph at the equivalence point. The analyte (titrand) is the solution with an unknown molarity. Substances such as phenolphthalein, which can. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Often, an indicator is. Acid Base Titration Indicator Example.